Yescarta®▼ (axicabtagene ciloleucel) is recommended for use within the Cancer Drugs Fund for treatment of adult patients with DLBCL who are eligible for ASCT, or if they have relapsed within 12 months after, or are refractory to, first-line chemoimmunotherapy.
Tecartus®▼ (brexucabtagene autoleucel) has been granted conditional marketing authorisation and is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL) after two or more lines of systemic therapy including a Bruton's tyrosine kinase (BTK) inhibitor.
Tecartus® is additionally indicated for the treatment of adult patients 26 years of age and above with relapsed or refractory B-cell precursor acute lymphoblastic leukaemia (ALL).

NHS England (including Northern Ireland and Wales)
Second-line Yescarta® for: diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBCL) in adults that relapse within 12 months from completion of, or is refractory to, first-line chemoimmunotherapy1

NHS Scotland eligibility criteria
Second-line Yescarta® for: diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBCL) in adults that relapse within 12 months from completion of, or is refractory to, first-line chemoimmunotherapy2

NHS England (including Northern Ireland and Wales)
Third-line or more Yescarta® for: Relapsed or Refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL), in adults after two or more lines of systemic therapy1

NHS Scotland eligibility criteria
Third-line or more Yescarta® for: Relapsed or Refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL), in adults after two or more lines of systemic therapy2

NHS England (including Northern Ireland and Wales)
MCL: Tecartus® for: Relapsed or Refractory (R/R) mantle cell lymphoma (MCL) in adults after two or more lines of systemic therapy including a Bruton tyrosine kinase (BTK) inhibitor1

NHS Scotland eligibility criteria
MCL: Tecartus® for: Relapsed or Refractory (R/R) mantle cell lymphoma (MCL) in adults after two or more lines of systemic therapy including a Bruton tyrosine kinase (BTK) inhibitor3

NHS England (including Northern Ireland and Wales)
ALL: Tecartus® for: adult patients 26 years of age and above with relapsed or refractory B-cell precursor acute lymphoblastic leukaemia (ALL).1
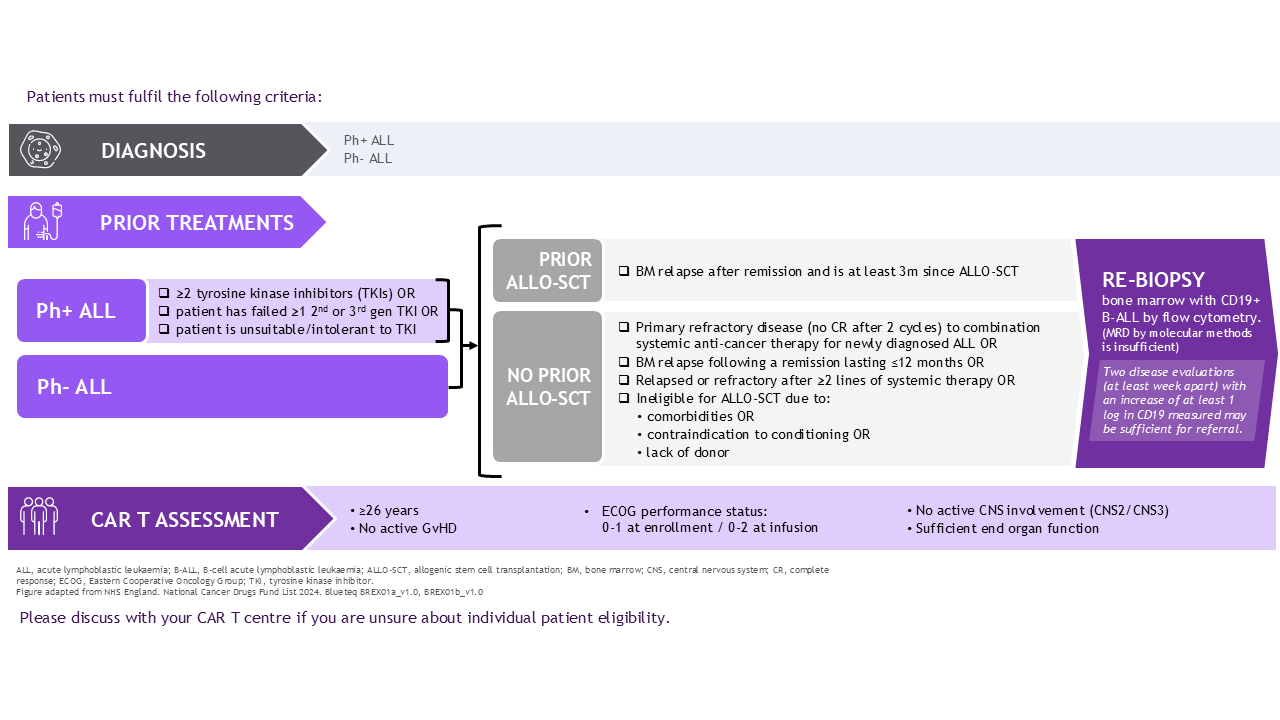
NHS Scotland eligibility criteria
ALL: Tecartus® for: adult patients 26 years of age and above with relapsed or refractory B-cell precursor acute lymphoblastic leukaemia (ALL).4

View Authorised Treatment Centres
April 2025 UKI-YES-0066
Adverse events should be reported
Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or via the Yellow Card app (download from the Apple App Store or Google Play Store). Adverse events should also be reported to Gilead to [email protected] or +44 (0) 1223 897500.